Nature of C - X Bond in Haloalkanes
Nature of C - X Bond in Haloalkanes: Overview
This topic covers concepts, such as, Polarity of Carbon-Halogen Bond in Haloalkanes, Bond Length of Carbon-Halogen Bond in Haloalkanes, Bond Enthalpy of Carbon-Halogen Bond in Haloalkanes & Dipole Moment of Carbon-Halogen Bond in Haloalkanes etc.
Important Questions on Nature of C - X Bond in Haloalkanes
Which is not the correct statement about the below molecule?
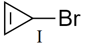
For a given alkyl group, the reactivity order of halides in reaction is
The CORRECT statement for is
The most polar compound among the following is:
is a gas, is a liquid, and are solids at room temperature. The highest bond enthalpy is observed in ______
The decreasing order of bond enthalpies of the following alkyl halides is:
(i)
(ii)
(iii)
(iv)
The correct order of dipole moment is-
Which of them has highest dipole moment ?
The order of dipole moment of the following molecules is :
Correct bond length order for carbon-halogen bond :-
Which of the following compound has zero dipole moment?
In which of the following pairs first has higher dipole moment than second?
In haloalkanes, the carbon-halogen bond enthalpy decreases from
In haloalkanes, the carbon-halogen bond length increases from
In a carbon-halogen bond of haloalkanes, the electron density is shifted more towards
Which of the following organic compounds unable to give precipitation after reaction with silver nitrate ()?
From which of the following sequences does the reactivity of alkyl halides in the nucleophilic substitution reaction follows:
Consider the following haloalkanes
The correct sequence of increasing order of dipole moments is
Which of the following are arranged in the decreasing order of dipole moment?
Which of the following molecules have zero dipole moment?
(I) gauche conformation of 1, 2-dibromoethane.
(II) anti conformation of 1, 2-dibromoethane.
(III) trans-1, 4-dibromocyclohexane
(IV) cis-1, 4-dibromocylohexane
(V) tetrabromomethane.
(VI) 1, 1-dibromocyclohexane
